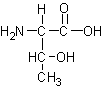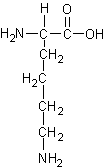Answer the following.
Please avoid writing the Great American Novel – none of these questions
require more than a paragraph or so.
Show all calculations for maximum credit. Good luck!
1)
A hemorrhagic fever of unknown origin
has broken out in
a. People
infected with the virus begin to produce large amounts of a protein, which
destroys collagen in the connective tissues.
You have isolated a large amount of the protein and are attempting to
determine the amino acid sequence. The protein is cleaved into two fragments
by treatment with cyanogen bromide (CNBr).
You have isolated one of the
fragments, which can itself be cleaved by the proteases trypsin and chymotrypsin
into the following fragments:
|
Trypsin |
Chymotrypsin |
|
DHM |
DRNELF |
|
DNFQVTK |
NTLCKDNF |
|
GLGYDR |
TCKDHM |
|
NTLCK |
QVTKGLGY |
|
NELFTCK |
|
For 8 points and the
salvation of humanity, what is the amino acid sequence of the fragment?
NTLCKDNFQVTKGLGYDRNELFTCKDHM
For 1 extra point,
would this fragment be at the amino (NH2) terminus or the carboxy
(COOH) terminus of the intact protein?
For 2 extra points, why?
Amino
terminus. The protein cleaved into two fragments; this fragment ends with a methionine
indicating an internal cleavage site. CNBr
cleaves after
2)
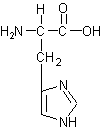
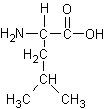
Identify the following four amino acids
by name(3 pts each).and one-letter code (2 pts each).
|
|
|
|
Name: _Threonine_____ Code:
_ |
Name: __Histidine____ Code:
__H_______ |
|
|
|
|
Name: __Leucine______ Code: ___L________ |
Name: __Lysine______ Code: ___K______ |
|
|
|
3)
Define or describe the following
terms. One or a few sentences should
suffice – do not write the Great American Novel. (5 pts each)
a. Hydrogen
Bond
Bond
between a hydrogen atom covalently bound to an electronegative atom and a
second electronegative atom. Relatively
weak; highly directional.
b. Gel
Filtration Chromatography
Separation
based on molecular size. Beads contain
pores of restricted size, allowing smaller molecules to “see” a greater
effective volume than larger molecules.
c. Entropy
S = k*lnW,
where k is Boltzmann’sconstant and W is the number of possible states the
system can occupy.
d. Beer’s
Law (hint – ultraviolet)
I =
Or
Where e is
the extinction coefficient, c is the concentration, and l is the path length
e. Peptide
Bond
Condensation
product between carboxyl group of one amino acid and the amino group of
another. Partially double bond in nature
and cannot rotate
f.
Secondary Structure
Local folding of
a protein into a set of characteristic stuctures (a-helix, b-sheet, etc.) based on hydrogen bonds formed
between backbone atoms
(carbonyl oxygens and
amide nitrogens).
g. Edman
Degradation (hint: Phenyl Isothiocyanate)
Method used
in chemical sequencing of a protein. PITC
reacts quantitatively with the amino terminus of a peptide to cleave off the terminal
amino acid. Reaction can be repeated to cleave
and identify one amino acid at a time.
4)
Mustard gas, Cl-CH2-CH2-S-CH2-CH2-Cl
(also known as HS or Yperite) is an inexpensive, easily manufactured and highly
effective blistering agent. Although
fatalities are rare, victims frequently suffer blindness, agonizing burns, long
term respiratory damage, permanent incapacitation, and drastically reduced
lifespans. The agent is also persistent
- casualties can result from individuals taking shelter in contaminated
soil. You have discovered an enzyme from
a rare species of Bolivian poison dart frog that inactivates mustard gas,
converting it into an effective deodorant.
The battlefield uses are obvious.
Your initial extract contains several
proteins with the following properties:
|
Protein |
Molecular Weight |
pI |
Shape |
|
A |
83,000 |
5.1 |
Globular |
|
B |
12,000 |
7.5 |
Globular |
|
C |
41,000 |
7.9 |
Globular |
|
D |
33,000 |
5.4 |
Globular |
|
E |
33,000 |
5.1 |
Rod-shaped |
|
F |
83,000 |
8.2 |
Globular |
a. I apply the
mixture to a chromatographic column containing an an anion
exchanger (Diethylaminoethylcellulose) buffered at pH 6.5. Based on your knowledge
of pH and pI, and of ion-exchange chromatography, which proteins will stick to
the column at this pH? (5 pts)
B, C and F have a negative charge
at pH 6.5 and will stick to the column
b. SNEAKY QUESTION ALERT! We talked about gel electrophoresis of
proteins in the presence and absence of sodium dodecyl sulfate. If I electrophorese the mixture in the absence of
c. The purified
inhibitor is electrophoresed on a gel.
Lane 1 contains Protein E
plus Sodium Dodecyl Sulfate (SDS). Lane 2 contains Protein E, SDS, and b-mercaptoethanol.
|
b-mercaptoethanol |
|
HS-CH2-CH2-OH |
The gel is seen below:
|
1 2
|
1: SDS, - b-ME 2. SDS, + b-ME |
What does the fact that lane 2 contains 1 band of protein while lane 3 contains 3 bands signify? (i.e. What is the effect of b-mercaptoethanol and what does it tell us about Protein E?) (Straight off the practice test – should be a freebie!) (4 pts)
Protein E
contains three separate chains linked by disulfides. These disulfides are reduced by BME, allowing
the chains to separate.
5)
For
the conversion of phosphoenolpyruvate to pyruvate:
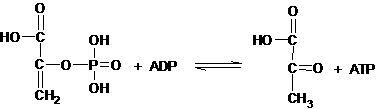
under normal cellular conditions, DG ≈ -42 kJ/mol.
a. Would you expect the reaction to
spontaneously proceed to the right
or to the left under cellular
conditions? Why? (5 pts)
To the right; DG
< 0
b. What is DGo’ Keq at 37oC (5pts)
(TYPO ON TEST)
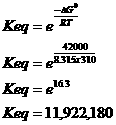
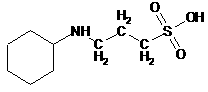
6)
CAPS
(3-(Cyclohexylamino)-1-propanesulfonic acid) is a common biochemical buffer
that is frequently used when experimental conditions require a high pH.
|
|
CAPS:
Molecular
Weight: 221.32 g/mol pKa 10.4 |
I wish to make 3 liters of 30 mM (0.03 molar)
CAPS buffer pH 11.0. I have a jar
of solid CAPS free acid, a stock solution of 10 M NaOH, a 4-liter graduated
cylinder, and all the distilled water I can possibly want. Tell me how to make the buffer. (10 pts)
(a)
3l * .03
(b)
pH =
11 =
0.6 = log[base]/[acid]
3.
[base]+[acid]
= .03 M
4.
[acid]
= 0.006M
[base]
= .03 - .006 = 0.024M
Need
to add .024M NaOH
(.024 M/ 10M) * 3l =
.0072l = 7.
Water to 3l
7)
The town of
(about life size for adult female)
You
have just been sprayed in the eyes.
Calculate the pH of the solution while you wait for the blinding pain to
stop. Assume that the pKa for CH3COOH is 4.76 . (7
pts)
Ka = [H+]{Ac-]/[HAc]
Ka = [H+]
[H+] = (Ka*[
[H+] = .00246M
pH = -log[H+] =
Useful Physical Constants
|
Constant |
Value |
|
Avagadro’s Number |
6.02 * 1023 |
|
Boltzmann’s Constant |
1.3807 * 10-23 JK-1 |
|
Charge on electron |
-1.602 x 10-19 coulomb |
|
Gas Constant (R) |
8.314 JK-1mol-1 |
|
Faraday’s Constant |
96,485 JV-1mol-1 |
|
k = 1/(4pe) |
8.99 x 109 Nm2coulomb-2 |
|
ln(x) |
2.303 log (x) |
|
Dielectric constant of water |
78.54 |